Challenge
The project of the medical device in question was commissioned to the order of a partner from Denmark with research and development offices in Poland. When the order was accepted, an advanced, proof-of-concept of the device was made, which, apart from the mechanical part, consisted of two components: the user interface board and the motor-driver board for a certain pump. Although the prototype had most of the functionality assumed, it had “teething problems”, so-called, such as problems with the current flow sensor. In addition, there were documentary deficiencies that de facto prevented further development of the project and thus, certification and serial production, in addition. For this reason, the Customer decided to transfer the board design to the Altium tool and create a dedicated software for it “from scratch”.
Due to the required device Safety Class II, classification for medical devices, the basic requirement of the Customer was to take into account the provisions of safety standards during manufacture, including PN-EN 60601 for hardware and PN-EN 62304 for software. Moreover, there were also technological requirements, related to the electro-mechanical components used in the device, as well as the need to meet the quality standards set by the Customer himself.
“The project was a challenge because we were dealing with a physically existing solution that contained many unusual, proprietary solutions which – as it turned out – had not been documented by the previous supplier. In order to recreate the original technical assumptions, it was necessary to audit the existing solutions and obtain some specific requirements, using the reverse engineering method. In addition, the project team collided with a unique, Scandinavian work culture, which – apart from technical issues – was also a big challenge from the point of view of project management.”
Jakub, Project Manager
In the early phase of the project, the team verified the existing hardware solutions submitted by the Customer. As a result, it was concluded that while the hardware platform provided could be used, it was not possible to maintain the existing source code, due to documentation deficiencies. The deficiencies shown made it impossible to prepare the final technical documentation, in order to certify the device.
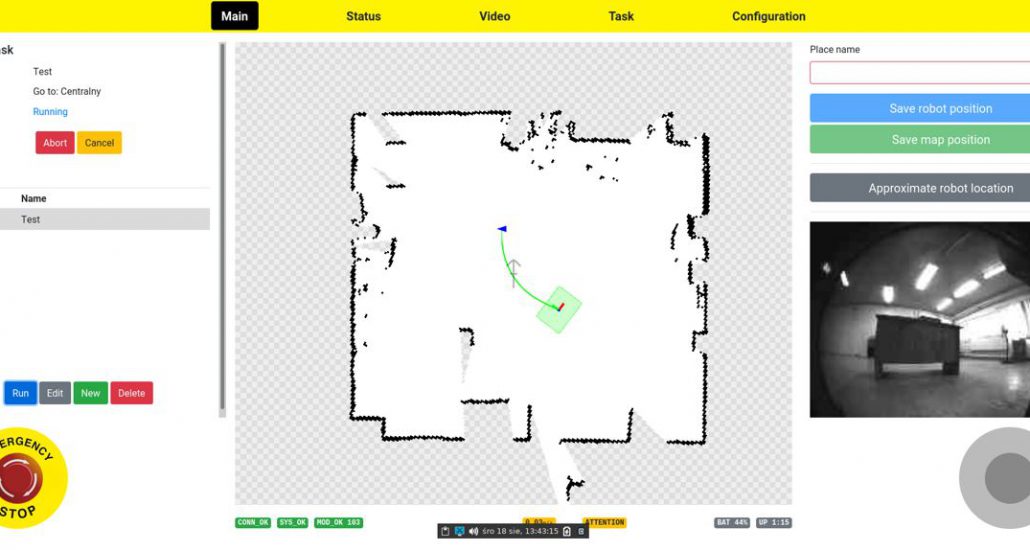
Therefore, the Customer was offered migration and significant correction of the hardware design, including processes, for both boards and the development of new software for both boards. The approved plan was implemented in several successive stages. Additionally, on the basis of the Customer’s guidelines, a new service application was developed which enabled the preview of the key parameters of the device, in real time and a full software update.
Results & Benefits
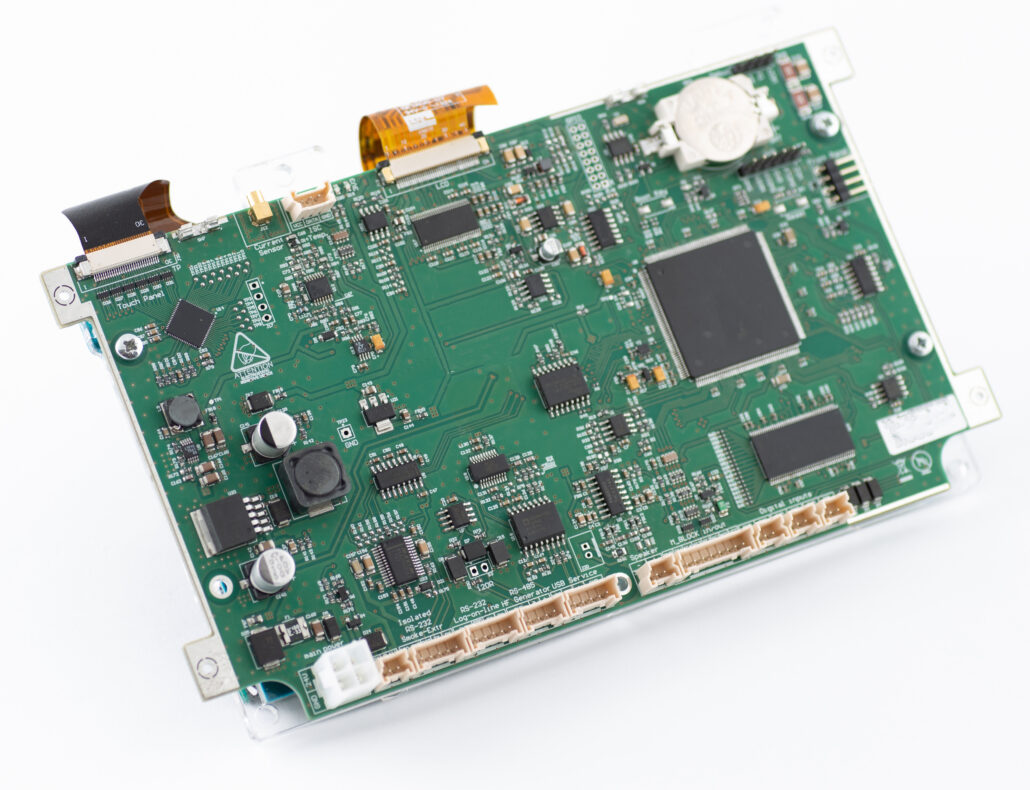
As part of the project, the team delivered two, key, fully functional electronic modules: a user interface board and a pump motor-driver board. The most spectacular achievement was the improvement of the key performance parameter of the device, which, from the business point of view, is the competitive advantage of the product. It is worth adding that, apart from the delivery of prototypes and full technical documentation of the equipment and a source code, the Customer was supported at every stage of the manufacturing process, as also during the creation of the documentation necessary, to carry out the certification process of the device, as well as its validation by the certification body.
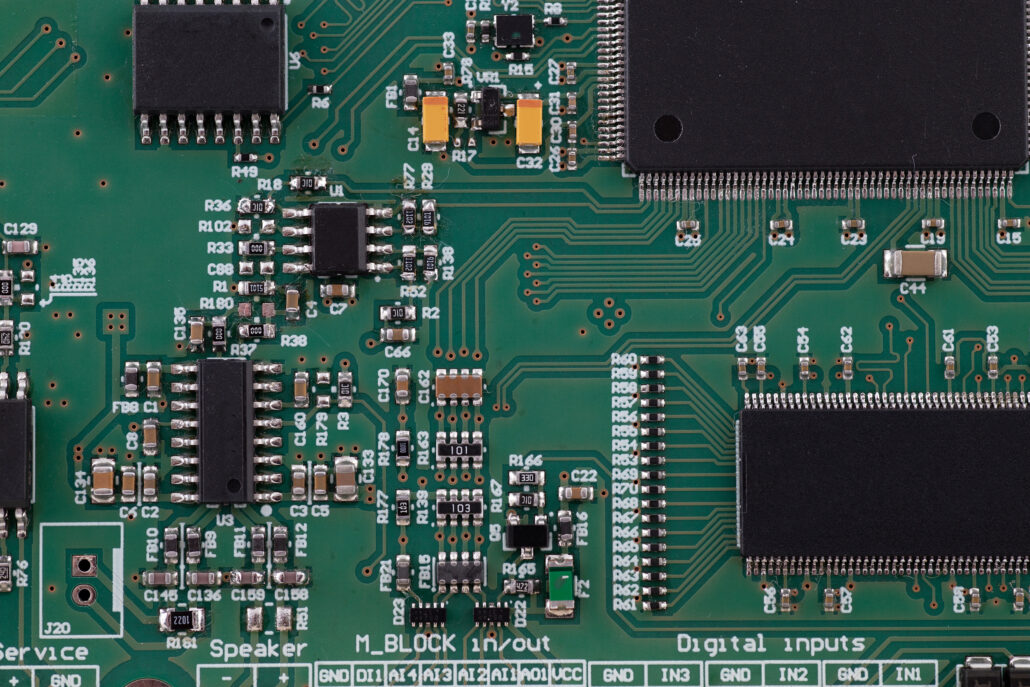
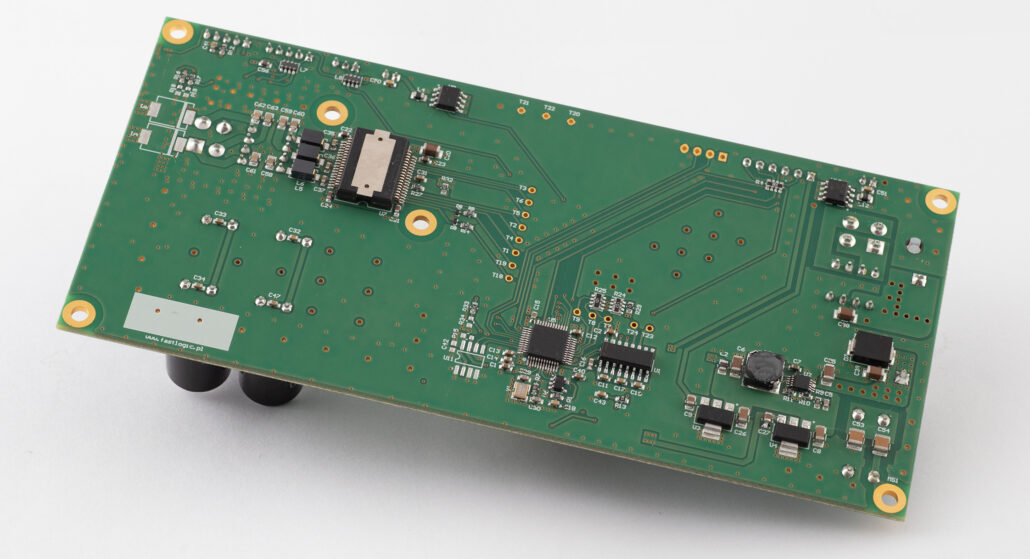
- Development of two, 4-layer PCBs, in accordance with the requirements of PN-EN 60601, preparation of prototypes, BOM optimisation.
- Carrying out tests that led to improvements in a number of performance parameters of the device, including cooling of the engine driver/BLDC.
- Development of bootloader and firmware for two processors of the STM32 family and an algorithm for device chain programming, based on the YModem protocol.
- Development of PC applications.
- Development of the documentation for the Software Validation in accordance with the requirements of PN-EN 62304
Volume and coverage
The product was implemented in the United States, with the annual production volume being some several hundred units of the device.